How Scientists Are Finally Tackling Drug Development's Biggest Problem
- Yajush Gupta
- Sep 4, 2025
- 5 min read
Updated: Sep 9, 2025
Why cardiac organoids mean your next medication will be safer: 3D human heart tissue revolutionizes pharmaceutical testing
What's happening: Laboratories across Europe and North America are replacing traditional animal testing with 3D cardiac organoids made from human stem cells that beat like real hearts and detect drug risks that conventional methods miss.
Why this matters: Cardiovascular safety issues remain the leading cause of drug development failures, with disasters like Vioxx's £4.85 billion settlement showing the human and financial cost of inadequate testing methods.
The numbers tell a sobering story. Despite decades of advances in pharmaceutical research, cardiovascular safety issues continue to halt promising treatments and reshape entire development programmes. Recent breakthroughs suggest we may finally understand why - and more importantly, how to fix it.
Cardiac organoids emerge
Every few years, the pharmaceutical industry faces another cardiac safety crisis. Drugs that sailed through preclinical testing suddenly reveal cardiovascular risks in human trials or, worse, after reaching patients.
The most striking example remains Vioxx. In 2004, Merck withdrew the arthritis medication after discovering it increased heart attack and stroke risks. The settlement ultimately cost $4.85 billion, but the human cost was far greater. Every few years the pharmaceutical industry faces another cardiac safety crisis. Drugs that sailed through preclinical testing suddenly reveal cardiovascular risks in human trials or after reaching patients.
What made Vioxx particularly troubling was not just its impact but how predictable the disaster seems in hindsight. The drug passed traditional preclinical cardiac safety tests. Animal models showed no concerning cardiovascular signals. Yet its mechanism involving COX-2 inhibition and prostaglandin balance created risks these established methods could not detect.
Two decades later, the fundamental approach has not changed much.
The issue is not testing more rigorously - it is testing more accurately. Traditional cardiac safety assessment faces what researchers call a recognition problem. Current models do not represent human cardiac physiology precisely enough to predict human responses.
Consider dofetilide, a medication known to trigger Torsades de Pointes - a potentially fatal heart rhythm disorder. Standard 2D cell cultures and animal studies often miss this risk because they cannot recreate the specific electrophysiological environment where the arrhythmia develops.
The disconnect is fundamental:
Flat cell cultures bear little resemblance to three-dimensional heart tissue
Animal models, while more sophisticated, fail to capture human-specific cardiac responses
These methods persist not because they are optimal but because they are established
When testing systems misrepresent the biology they are meant to evaluate, the solution is not more testing - it is better testing.
A different approach emerges
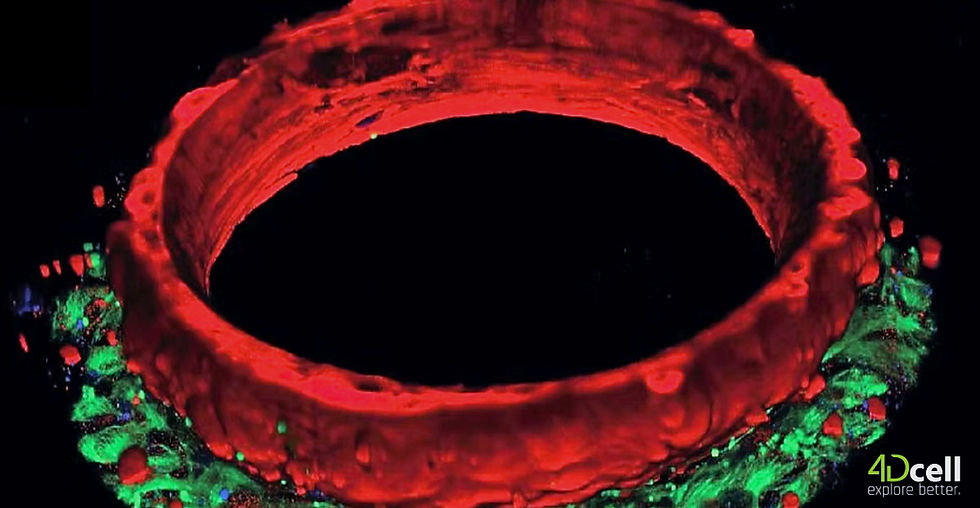
Laboratories across Europe and North America are shifting their thinking. Instead of approximating human heart function, scientists are replicating it using 3D cardiac organoids derived from human induced pluripotent stem cells. These studies are species-dependent, so using human cells is crucial.
These organoids are simplified models, but each consists of human cardiac tissue arranged in ring-shaped structures that beat with physiological rhythm and respond to compounds in ways that reflect real human heart behavior. At 4DCell, in collaboration with Prof. Jean Sébastien Hulot at INSERM, we focused on addressing practical challenges that have limited adoption of this technology. The result is SmartHeart, a platform combining several key innovations:
Architectural precision Proprietary hydrogel microwells create controlled microenvironment guiding consistent cardiac tissue formation. This addresses reproducibility across experiments and laboratories.
Automated analysis Machine learning algorithms capture and analyse key cardiac readouts, including contractility, action potentials, and calcium transients. This eliminates subjective interpretation and generates the quantitative data required for regulatory submissions.
Biological authenticity Unlike traditional cell cultures these organoids express mature cardiac markers and demonstrate electrophysiological responses closely mirroring human heart tissue.
The regulatory landscape is shifting
The movement toward human-relevant testing reflects both scientific necessity and regulatory evolution. European agencies are increasingly prioritizing animal-free testing approaches, while FDA guidelines place growing emphasis on human-relevant models. In April 2025, the FDA announced a plan to phase out animal testing requirements for monoclonal antibodies and other drugs, replacing them with more effective, human-relevant methods.
Subsequently, in July 2025, the NIH declared it would no longer develop new funding opportunities focused exclusively on animal models of human disease, encouraging broader approaches that include New Approach Methodologies (NAMs). The CiPA (Comprehensive in vitro Proarrhythmia Assay) initiative specifically calls for testing approaches that better predict human cardiac responses.
This regulatory shift acknowledges what many researchers have long suspected: when testing methods consistently fail to predict human responses, the problem isn't insufficient testing, it's inadequate models.
Evidence of progress
Recent peer-reviewed research demonstrates that 3D cardiac organoids can detect cardiac liabilities that traditional models miss¹. These platforms identify mechanisms that animal studies can't capture and provide dose-response relationships that correlate more closely with human clinical data. Perhaps most importantly, they can identify the specific cardiac risks that derail development programs:
QT interval prolongation and pro-arrhythmic potential
Early afterdepolarisations and conduction abnormalities
Contractility impairments and rhythm disturbances
Ion channel dysfunction across multiple pathways
This comprehensive assessment capability addresses the early warning gap that has plagued drug development pipelines.
Beyond risk mitigation
The implications extend beyond avoiding catastrophic failures like Vioxx. Early, accurate detection of cardiac liabilities enables strategic decision-making when modifications are still feasible and cost-effective. Development teams can prioritise compounds based on human-relevant data, make informed go/no-go decisions earlier in the process, and approach regulatory submissions with more predictive evidence.
The technology transforms cardiac safety assessment from a late-stage hurdle into an integrated part of compound optimisation.Several organisations have begun integrating these approaches into their development pipelines. Early adopters report identifying cardiac risks that traditional screening methods missed, making development decisions with greater confidence, and engaging with regulators using human-relevant data. The market increasingly demands more complete solutions that recapitulate in vivo mechanisms, such as multi-organ integration (e.g., liver-heart systems), to further strengthen this human-relevant approach.
Cardiac Organoids evolve
The technology exists, and regulatory pathways are improving, as seen with recent FDA guidance. What remains is broader implementation and the effort to fully establish New Approach Methodologies (NAMs) in ways that allow industry to confidently move beyond familiar but limited models toward testing systems that more accurately reflect human biology. The pharmaceutical industry's cardiac safety problem isn't unsolvable, it's been a modeling problem disguised as a biology problem.
For the first time in decades, we have the tools to test drugs using actual human heart tissue, arranged in structures that beat and respond like the hearts of future patients.
SmartHeart is being adopted by pharmaceutical and biotechnology companies worldwide. For technical specifications validation data or implementation guidance: Contact us to start the conversation.
References
1. Chi, K. Revolution dawning in cardiotoxicity testing. Nature Reviews Drug Discovery 12, 565–567 (2013)
2. Gintant, G. et al. Evolution of strategies to improve preclinical cardiac safety testing. Nature Reviews Drug Discovery 15, 357–372 (2016)
This article draws on research from 4Dcell, INSERM, and peer-reviewed studies published in Nature Reviews Drug Discovery.
Contact: contact@4dcell.com
Resources: 4dcell.com




Grant Pharmacy makes it easy to order Amoxicillin online with trusted service and quick delivery.
Sanford Pharmacy is more than just a place to fill prescriptions – it is a trusted partner in your healthcare journey. Dedicated to offering quality medicines and reliable services, Sanford Pharmacy combines professional care with a personal touch.