> REVOLUTIONIZING CELL CULTURE
by implementing adhesive micropatterns to control the microenvironment
> REVOLUTIONIZING CELL CULTURE
by implementing adhesive micropatterns to control the microenvironment
Heiva Le Blay, Serena Pavoni
May 2017
> ABSTRACT
The control of the cellular microenvironment allows to understand the different cellular mechanisms that underlie cell-to-cell and cell-ECM interactions, which are essential to medical research (cancer research, regenerative medicine, drug discovery…). In conventional 2D cultures, cells are randomly seeded and grow without a specific organization. This in vitro system is commonly used in research to more easily obtain a population of cells maintained on a flat and homogeneous substrate of adhesion. Major drawbacks of these adherent cultures are the difficulty to control environmental parameters for individual cells and the impossibility to recapitulate multicellular architectures, tissue-tissue interfaces and the physicochemical microenvironments of in vivo conditions.
Organs and tissues are characterized by interacting cells organized in a 3D fashion, which in turn reflects a specific cell configuration. The absence of physiologically relevant cell conformation strongly impacts on cell mechanisms and behavior. Biology research needs more control over cell culture methods allowing the reconstitution of tissue-like conditions in vitro.
Recent advances in cell culture biology consist in the treatment of specific substrates to create adhesive surface, in order to obtain a better control over spatial organization. Surface treatments contribute making cell culture conditions less artefactual and closer to physiological conditions. The use of patterns of adhesive proteins at micrometer scale, also known as micropatterns, offers the possibility to better control cell environment and thus the cytoskeleton architecture, as well as polarity, migration, division, growth, and differentiation of cells.
This review outlines recent applications for cell culture thanks to the use of adhesive micropatterns.
> INTRODUCTION
Although the first micropatterning methods for manipulating cell adhesion patterns have been developed more than 40 years ago (Carter, 1967; Harris, 1973), they have been commercially available since 2009 and recently became an essential tool to cell biology laboratories.
There are many different ways to create micropatterns which can be separated into two categories: the indirect cellular patterning and the direct patterning. Falconnet and colleagues (2006) present through their review how to produce engineered micropatterned surface for cell culture with all the major techniques described in the literature.
Here, the focus of this review is on the principal use of micropatterns depending on the shape of the micropattern, which allows to control the shape and spread of seeded cells mimicking the physiological spatial confinement (Singhvi et al., 1994), which enables to regulate the cytoplasmic organization and thus cellular functions (Pitaval, Tseng, Bornens, & Théry, 2010; Polte, 2004; Roca-Cusachs et al., 2008; Tan et al., 2003).
Therefore, the choice of micropattern geometry is critical and needs to be adapted according to the purpose of the biology research. Micropatterns constitute an easy way to standardize cells experiments.
The main applications of micropatterns described through research literature are the study of cellular functions (migration, differentiation, polarity, chirality…), the clinical research and screening test, as well as the investigation of the neuronal network.
> STUDY OF CELLULAR FUNCTION
> Shape control and spatial confinement of cells
The first main advantage of micropatterns compared to the Petri dish is the control of the cell shape. Indeed, when the cell adheres to the micropattern, it adapts and takes its shape according to the micropattern geometry (e.g. round, square, elongated, etc.). For example, in an “L” shape, the cell attaches to the adhesive micropattern then spreads along the bars, and adopts the triangle shape while in a “U” shape, the cell adopts a squared shape (Bornens et al. 2003). Finally, depending on the cellular shape adopted it is possible to observe nucleus orientation and deformation. In a round micropattern, cell and nucleus adopt circular conformation while into square or rectangular geometry, the nucleus isn’t circular anymore and get closer to an ellipse shape. Finally with elongated rectangle, the cell is forced to adjust the geometry of its shapes in an elongated, aligned and oriented manner while the nucleus undergo the same deformation (Khatau et al., 2009; Versaevel et al., 2012; Zhang et al., 2016).
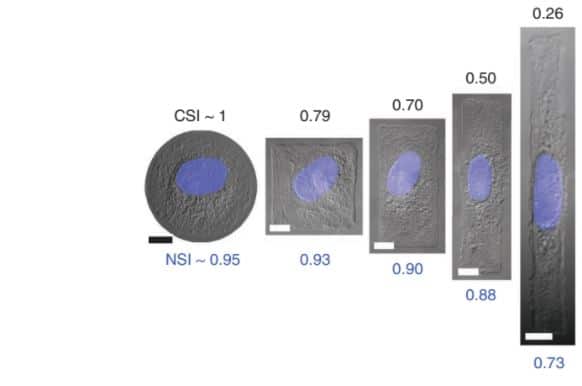
In addition, it is possible to study the mechanisms implicated in the control of the cells, the length and orientation. When using linear strips micropatterned, it can vary. For example, fibroblasts adopt an elongated and oriented shape along the strip’s length when grown on a strip while epithelial cells do not seem to regulate their cell length to this extent (Levina et al., 2001).
> Cell migration
It is interesting to study the cell migration according to the shape of micropattern. Cell migration on thin micropatterned tracks recapitulates better the in situ situation than cell migration on homogeneous and large surfaces. Experiments showed that cells plated on wide tracks of fibronectin migrate in a similar manner as cells grown on Petri dishes, with their centrosome oriented in front of the nucleus and towards the lamellipodia. By contrast, in cells confined to thin tracks, the centrosome is located in the back of their nucleus (Pouthas et al., 2008).
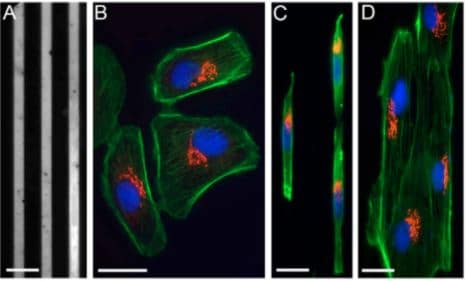
Fig.2 Cells on fibronectin-patterned lines are polarized. (A) Patterned, 6-μm thick fibronectin lines and Pll–g-PEG. (B) Bsc1 cells on a standard glass coverslip labelled for the actin network (green), the Golgi complex (red) and the nucleus (blue). (C) Bsc1 cells labelled as in B, grown on 6-μm fibronectin lines. (D) Ptk2 cells labelled as in B, grown on 6-μm fibronectin lines.
Image extracted from Pouthas et al. 2008.
To study the migration of cells, dispositions of different shape of micropatterns have been thought of. It is possible to connect micropatterns in a linear or circular way, and to study the movement of the cells on the periodic micropatterns. The alignment of triangles connected to each other will guide sequential cell movement from one triangle to the next while disconnected triangles do not allow cell movement (Mahmud et al., 2009). Another study shows that on asymmetric micropatterns like triangles arranged in a line one after the other and separated by non-adherent gaps, the cell fluctuates from left to right inside the triangle before migrating in a directional manner suggesting that distance between the neighboring patterns influence the probability to reach the next adhesive area. For symmetrical micropatterns (e.g. symmetric fibronectin discs disposed one after the others without contact), the cell will migrate randomly without a preference for direction (Caballero et al., 2015).
Moreover, in a round micropattern, the angular cell movement can be calculated as a function of the number of confined cells in the micropatterns (Segerer et al., 2015).
Another example about the study of cell migration compares micropatterned squares with triangles disposed one after the others (without contact between the micropatterns). The findings show that the distance of cell migration on the symmetric squares is shorter than on the triangles even if each cell migrates on the next micropattern on the right side. Therefore, cells migration depends strongly on the micropattern shape and configuration (Kim et al., 2016).
> Cell division and cell polarity
The polarity of the actin cytoskeleton, i.e. the relative position of protrusive and contractile regions of the network, which is established in interphase in response to adhesive cues, is maintained during mitosis and orients the cell division axis (Théry & Bornens, 2006).
The triangle shape has been used (as well as the “L” shape) to determine the axis of division, which determines the future position of the daughter cells. It has been found that a favorite elongated orientation is along the axis of the hypotenuse during anaphase (Théry et al., 2005). Others letter shapes have been selected for the study of the cell division. For micropatterns with “O” and “Y” shapes, mitosis is multipolar (anomaly of mitosis due to a defect in the centrosome duplication), while for the shape “H”, mitosis is bipolar (Kwon et al., 2008). Micropatterns can control cell division, which is important in the case of cancer study (see clinical application) or during 3D tissues organization.
Fink and colleagues use a combination of micro-manipulation tools on human dividing cells to address the role of physical parameters of the microenvironment in controlling the cell division axis. They combined adhesive micropatterns with laser ablation to remove specific retraction fibers. Specifically, they grew HeLa cells on either bar or asymmetric cross-shaped patterns, and allowed cells to enter mitosis and the spindle to align with the adhesive pattern (with the spindle aligning with the long axis of the adhesive shape in each case). Once the spindles were aligned, the retraction fibers adjacent to each end of the spindle were cut by laser ablation. On the cross-shaped pattern, spindles rotated and aligned with the perpendicular axis dictated by the remaining retraction fibers. In contrast, on the bar-shaped pattern, where no retraction fibers remained, no significant spindle reorientation was observed (Fink et al., 2011).
Crucially, these experiments demonstrate that retraction fibers, in addition to be the remnants of interphase cell polarity, are also actively involved in spindle orientation. Moreover, the finding that spindles reorient once retraction fibers are cut demonstrates that the spindle is not simply following shape/force cues laid down during interphase but is instead continuing to monitor the adhesive environment and adjusting its orientation accordingly. The comparison with two different micropatterns shapes allows to deduce that external forces control mitotic spindle positioning.
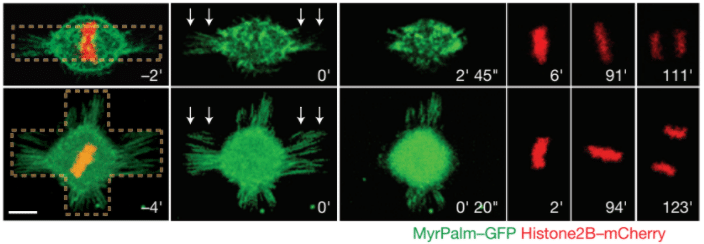
Fig.3 – Mitotic HeLa cells on bar-shaped (top row) or asymmetrical cross shaped (bottom row) micropatterns. White arrows represent the laser ablation. MyrPalm–GFP (green) and histone2B–mCherry (red) allow the visualization of plasma membrane/retraction fibres (green) and chromosomes (red). Scale bar 10 µm.
Image extracted from Fink et al. 2011.
> Cell differentiation
Micropatterning methods can be useful to allow the reconstitution of tissue-like conditions for in vitro cell culture and the control of cell shape has an important role in allowing the control of cell reprogramming as well as in the study of stem cell differentiation (Tseng et al., 2014).
For example, Connelly et al. employ a circular micropatterned surface in order to identify the signaling pathways involved during human epidermal stem cells differentiation. This study shows that keratinocyte terminal differentiation is dependent on cell shape rather than ECM composition or concentration. These results point out the importance of physical parameters during the transduction of extrinsic stimuli into transcriptional response, which determine stem cell niches fate (Connelly et al., 2010).
McBeath and colleagues established that cell shape and cytoskeletal arrangement influence cell fate determination. Human mesenchymal stem cells (hMSCs) on a micropatterned island differentiate into adipocytes or osteoblasts depending on the size of the island and on the amount of cell spreading. They show that the type of differentiation is dependent upon activation of the RhoA signaling pathway, the activation of which is triggered by cell shape (McBeath et al., 2004).
Another specific example, regarding mesenchymal stem cell differentiation compares the effect of three pentagonal symmetry shapes of the same area on MSC lineage commitment. In this study, Kilian et al. (2010) found a 62% of adipogenic profile for the shape that approximated a flower and the same percentage for the osteogenic profile in a shape that approximated a star. Regarding the pentagon shape, the percentage of the stem cells differentiation was approximately the same for both profiles (adipocytes and osteoblasts). These findings confirm the importance of geometric shape on cell differentiation mechanisms (Kilian et al., 2010).
> STUDY OF NEURONAL NETWORK
In a neuronal network, signal transmission between neurons occurs at specialized contact sites called synapses. Primary cultures of dissociated neurons in vitro are capable of forming functional synapses. (Fletcher et al. 1991, Fletcher et al. 1994). However, experiments (in vitro or in vivo) with complex neural circuits do not allow for control over a single synaptic contact. Engineered surfaces have shown promise as a powerful tool to investigate cell-cell communication at a synaptic level.
With the use of micropatterns, neuronal networks investigation has expanded its knowledge in the field of neurosciences. Designed micropatterns have been conceived to clearly show the neuron structure (soma, dendrites, and axons). The geometry of micropatterns must combine large (i.e. in the range 20–80µm in diameter) spots dedicated to cellular bodies (soma), and micrometer-sized stripes that ensure neuronal connection, the neurites (dendrites and axons) (Chang et al., 2008; Roth et al., 2012; Wyart et al., 2002).
Axons outgrowth can be controlled in non-adhesive surfaces with circular- or linear-shaped cell-adhesive micropatterns. Neurons form functional synaptic connection and neural circuit can be geometrically controlled.
Micropatterned grids for neuronal network are composed of node points which allow cell body attachment and a narrower line for neurite outgrowth. Grids micropatterns could geometrically guide geometrically controlled neural circuits with functional synaptic connections (Kwiat et al., 2012; Vogt et al., 2005; Wyart et al., 2002).
Furthermore, patterns composed by a round shape and 3 branched lines (separated from each other at an angle of 120 degrees), have the ability to control single neural cell soma position as well as the neurites growth on the arm and the formation of a cell-cell contact (Yoshida et al., 2016).
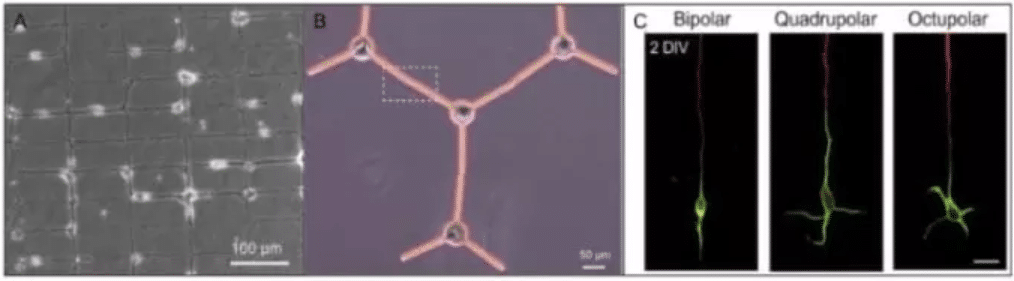
Fig.4 –Illustration of two different micropatterned neuronal networks (A and B) and single neuron polarizations in 3 distinct pattern geometries (C).A/ Typical image of a patterned neuronal network at DIV 7. Cell bodies adhere to the node points of the grid and connect along the pathways defined by the micropattern (Vogt et al., 2005). B/ Representative picture of an assembled microplates that had morphologically controlled single neural cells on them (somas on the circles and neurites on the arms, visible in black) (Yoshida et al., 2016). C/ Polarization of hippocampal neurons on micropatterns. Fluorescence micrographs of 2 DIV neurons on the three patterns stained with an axonal marker (tau-1; red) and a somatodendritic marker (MAP2; green). Scale bar, 20 μm. (H. Yamamoto et al., 2016).
Instead of discs connected through lines, polygons have been also used to control axon-dendrite polarity. As shown in Fig. 3C, the use of three pattern geometries (bipolar, quadrupolar, and octupolar) were compared to obtain different dendrite polarities of hippocampal patterned neurons (Yamamoto et al., 2012). The bipolar pattern is a geometry that is also frequently used in neuron patterning experiments and has been employed in a number of polarity control experiments. The octupolar pattern was intended to have “more than enough” paths for dendrites because a hippocampal neuron develops an average of 4.9 dendrites (Dotti et al. 1988). Indeed, on Fig.4C, we can count on the octupolar pattern about 5 dendrites.
Moreover, Yamamoto and colleagues showed that synapses formed by micropatterned neurons are physiologically functional and that the signal transmission occurs unidirectionally according to the geometry of the pattern. The development of synapses on micropatterned surfaces which allow for neuronal polarization is a first important step in assembling neuronal networks with a intended connectivity (H. Yamamoto et al., 2016).
In a 3D configuration, neurons can be manually placed into microwells connected with channels. This system allows neurons to develop neurites into microchannels. Moreover, neurites follow the channels into adjacent wells without any need for other promoting guiding cues. The physical cue of the channel and the chemical non-adhesive property of the agarose itself used by Krumpholz and colleagues allow neurites guidance. In this context, the dimensions of the microchannels seemed to play a critical role. In fact, experiments with different channel dimensions showed that smaller channels of width and height of 5-10 µm yield better guidance than larger ones of 30 µm width and height (Krumpholz et al., 2015).
Finally, well-controlled neuronal networks can be obtained in vitro by controlling cortical neurons migration with adhesive micropatterns. The method proposed by Lantoine et al. shows that neural soma confinement combined with the guidance of neurite elongation allow to generate reproducible and standardized neuronal networks which can be maintained in culture for up to 21 days, without altering their spatial organization. This system would facilitate the study of the individual neuron interactions with their neighbors or the behavior of neuronal networks after incubation with pharmacological agents (Lantoine et al., 2016).
> CLINICAL APPLICATION
Cell mechanics play a crucial role in many cellular functions that are critical during development and are altered during pathologies such as cancers. One of the biggest interests in clinical application is cancer research.
It has been shown that intracellular mechanics could be used to differentiate cancer cells from normal cells (Baker et al., 2010; Guo et al., 2014; Y. Li et al., 2012) . More precisely, Mandal and colleagues have developed an approach to map out the intracellular mechanical properties of living cells by combining micropatterning (with crossbow shape) and optical tweezers-based active microrheology. Mapping intracellular mechanics shows that metastatic breast cancer cells are softer than non tumorigenic cells and reveals differences in the distribution of intracellular viscoelasticity (Mandal et al., 2016).
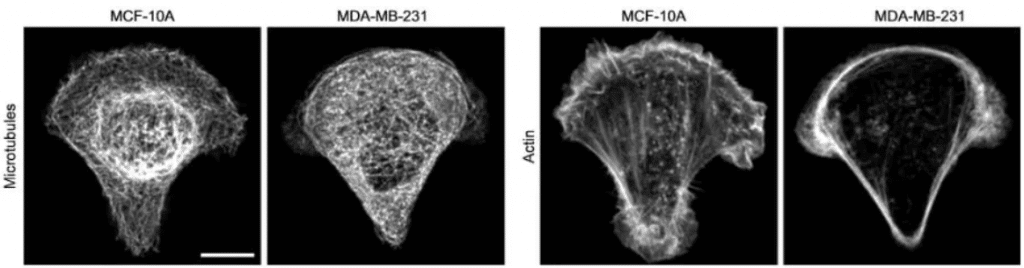
Fig.5 – In cancer cell MDA-MB-231, microtubules appear more homogeneously distributed and less bundled, and actin fibers are less abundant in the cell center and accumulate at the cell periphery. (Left: MCF-10A non tumorigenic cells; Right: MDA-MB-231 metastatic cells, scale bar 10 µm).
Image extracted from Mandal et al.2016
Left-Right (LR) asymmetry is a property which underlies the developmental mechanisms of numerous living organisms, including humans (Afzelius, 1976; R. Li & Bruce, 2009) and cell chirality is an intrinsic and well-conserved biological feature. The LR asymmetry of organs has been extensively studied but little is known at a cellular or a multicellular level.
Micropatterned cells exhibit a specific LR asymmetry depending on the cell’s specific phenotype. The comparison between circular and ring shapes is interesting in the study of cell chirality. Indeed, in round micropatterns, there is no chirality and cell migration is random, while in ring shapes, the cell chirality changes in the case of cancer cells (Wan et al., 2011).
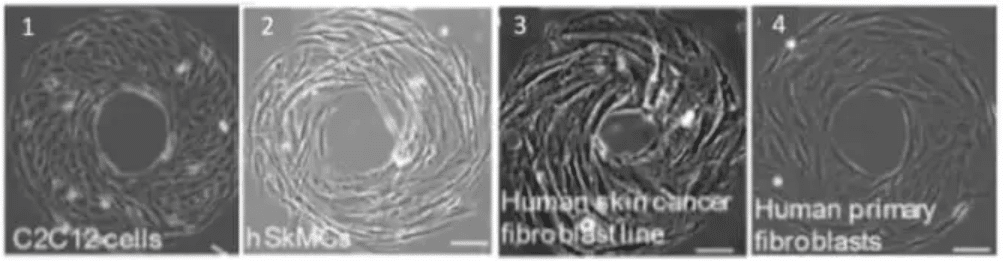
Fig.5 – Cell phenotype impact cell chirality. The mouse myoblast C2C12 cells (1) and human skeletal muscle cells (2) exhibit a counterclockwise (CCW). Human skin cancer fibroblast cell line shows a CCW alignment (3) while the correspondent healthy cell type (from human skin fibroblast) shows an opposite alignment (clockwise, CW). Scale bar 10 µm .
Image extracted from Wan et al. 2011
Furthermore, with “donut-like ring structures”, Rolli et al. demonstrate a break in symmetry between the behavior of cells along the outer convex boundary and along the inner concave boundary (Rolli et al., 2012).
An interesting characteristic feature of cancer cells is centrosome supernumerary, which can lead to the formation of multipolar spindles during mitosis. Multipolar divisions generate highly aneuploid cells that eventually die. To avoid these detrimental effects of multipolar divisions, cancer cells can form bipolar spindles with multiple centrosomes per pole, which will allow their survival. The geometry of the microenvironment affects the location of cortical cues that orient the spatial distribution of forces acting on the additional centrosomes. This, in turn, induces either centrosome coalescence (when the microenvironment is bipolar, such as on H-shaped micropatterns), or centrosome separation (when microenvironment is multipolar, e.g. on Y-shaped micropatterns) and, thus, eventually dictates the proportion of multipolar and bipolar spindles in cancer cells (Kwon et al., 2008). These results demonstrate that the cell microenvironment can either promote or hinder cancer progression, depending on the geometry of the microenvironment.
Another popular domain is regenerative medicine. The spatial orientation of nerve cells plays a pivotal role in nerve regeneration (Yang et al., 2005). Micropatterns has been used to guide sensory neurons showing that micropatterned substrate impacts neurons excitability. Benzina and colleagues created a micropattern design which consists in parallel lines containing in the middle a disc of variable diameter. This study shows that structural guidance on adhesive micropattern enables neuron polarization and provides a way to control axon guidance during development and regeneration mechanisms (Benzina et al., 2014).
Ohers micropatterns major applications are the development of cell-based sensors and drug discovery. Cell-based sensor devices contain living cells that monitor perturbations of the environment such as toxic or pathogenic agents (Jung et al., 2001; Park & Shuler, 2003; Ziegler, 1999). Cell-based assays and organ-on-chip systems in drug discovery are considered promising screening approaches, intermediate between genomic- or proteomic- in silico studies and in vivo animal models (Jung et al., 2001; Schwenk et al., 1983; Ziegler, 1999). The use of cell-based assays that mimic specific in vivo behavior is believed to decrease costs, provide better systems of screening, leading the drug discovery processes to be more predictive (Bhadriraju et al., 2002).
> CONCLUSION
In summary, micropatterns have become an essential tool in cell culture. The development of micropatterned systems revealed important insights into how the geometry of the microenvironment impacts on cellular physiology, from intracellular organization to multicellular morphogenesis.
Its conception has become easy and applicable to cell culture studies thanks to the generation of experimental kits which represent a more accessible, simple and cheaper way to use micropatterns in cell biology experiments. Today, cell culture is an accessible experimental tool because scientists contributed to develop systems for cell culture in vitro, making it more accessible.
This review covers the progress in cell culture on the use of micropatterns, showing there is a great potential for the future with the aim of solving a number of problems concerning the long term culture systems in vitro and the single cell arrays. Moreover, 3D cell cultures have recently been shown to be an essential tool in cell biology and in clinical applications. For instance, generation of whole organs in vitro (also called organoids) can recapitulate physiological developmental mechanisms as well as pathological disorders. The development of systems which enable the application of micropatterns to cultivate cells in a 3D configuration and for longer period of time represents the future of research. 3D micropatterned cells can reflect more closely the in vivo conditions in a physiological as well as in a pathological context.
> REFERENCES
Afzelius, B. a. (1976). A human syndrome caused by immotile cilia. Science, 193(4250), 317–319. https://doi.org/10.1126/science.1084576
Baker, E. L., Lu, J., Yu, D., Bonnecaze, R. T., & Zaman, M. H. (2010). Cancer cell stiffness: Integrated roles of three-dimensional matrix stiffness and transforming potential. Biophysical Journal, 99(7), 2048–2057. https://doi.org/10.1016/j.bpj.2010.07.051
Benzina, O., Cloitre, T., Martin, M., Raoul, C., Gergely, C., & Scamps, F. (2014). Morphology and intrinsic excitability of regenerating sensory and motor neurons grown on a line micropattern. PLoS ONE, 9(10), 1–10. https://doi.org/10.1371/journal.pone.0110687
Bhadriraju, K., & Chen, C. S. (2002). Engineering cellular microenvironments to improve cell-based drug testing. Drug Discovery Today, 7(11), 612–620. https://doi.org/10.1016/S1359-6446(02)02273-0
Caballero, D., Comelles, J., Piel, M., Voituriez, R., & Riveline, D. (2015). Ratchetaxis: Long-Range Directed Cell Migration by Local Cues. Trends in Cell Biology, 25(12), 815–827. https://doi.org/10.1016/j.tcb.2015.10.009
Carter. (1967). V. r., j. Retrieved from http://ac.els-cdn.com/0014482767902984/1-s2.0-0014482767902984-main.pdf?_tid=683f6cc8-8bbf-11e5-9ff7-00000aacb35d&acdnat=1447609170_fe21d472a587862989e6fe255334defd
Chang, W. C., & Sretavan, D. W. (2008). Novel high-resolution micropatterning for neuron culture using polylysine adsorption on a cell repellant, plasma-polymerized background. Langmuir, 24(22), 13048–13057. https://doi.org/10.1021/la8021479
Connelly, J. T., Gautrot, J. E., Trappmann, B., Tan, D. W.-M., Donati, G., Huck, W. T. S., & Watt, F. M. (2010). Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nature Cell Biology, 12(7), 711–718. https://doi.org/10.1038/ncb2074
Fink, J., Carpi, N., Betz, T., Bétard, A., Chebah, M., Azioune, A., … Piel, M. (2011). External forces control mitotic spindle positioning. Nature Cell Biology, 13(7), 771–8. https://doi.org/10.1038/ncb2269
Guo, Z., Hu, K., Sun, J., Zhang, T., Zhang, Q., Song, L., … Gu, N. (2014). Fabrication of hydrogel with cell adhesive micropatterns for mimicking the oriented tumor-associated extracellular matrix. ACS Applied Materials and Interfaces, 6(14), 10963–10968. https://doi.org/10.1021/am5023946
Harris, A. (1973). Behavior of cultured cells on substrata of variable adhesiveness. Experimental Cell Research, 77(1–2), 285–297. https://doi.org/10.1016/0014-4827(73)90579-X
Jung, D. R., Kapur, R., Adams, T., Giuliano, K. a, Mrksich, M., Craighead, H. G., & Taylor, D. L. (2001). Topographical and physicochemical modification of material surface to enable patterning of living cells. Critical Reviews in Biotechnology, 21(2), 111–154. https://doi.org/10.1080/20013891081700
Khatau, S. B., Hale, C. M., Stewart-Hutchinson, P. J., Patel, M. S., Stewart, C. L., Searson, P. C., … Wirtz, D. (2009). A perinuclear actin cap regulates nuclear shape. Proceedings of the National Academy of Sciences of the United States of America, 106(45), 19017–19022. https://doi.org/10.1073/pnas.0908686106
Kilian, K. a, Bugarija, B., Lahn, B. T., & Mrksich, M. (2010). Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America, 107(11), 4872–7. https://doi.org/10.1073/pnas.0903269107
Kim, S., Kim, M., Shin, Y., Eom, S., Lee, J., Shin, D.-M., … Han, D.-W. (2016). Cell Migration According to Shape of Graphene Oxide Micropatterns. Micromachines, 7(10), 186. https://doi.org/10.3390/mi7100186
Krumpholz, K., Rogal, J., El Hasni, A., Schnakenberg, U., Bräunig, P., & Bui-Göbbels, K. (2015). Agarose-Based Substrate Modification Technique for Chemical and Physical Guiding of Neurons In Vitro. ACS Applied Materials and Interfaces, 7(33), 18769–18777. https://doi.org/10.1021/acsami.5b05383
Kwiat, M., Elnathan, R., Pevzner, A., Peretz, A., Barak, B., Peretz, H., … Patolsky, F. (2012). Highly ordered large-scale neuronal networks of individual cells – Toward single cell to 3D nanowire intracellular interfaces. ACS Applied Materials and Interfaces, 4(7), 3542–3549. https://doi.org/10.1021/am300602e
Kwon, M., Godinho, S. A., Chandhok, N. S., Ganem, N. J., Azioune, A., Thery, M., & Pellman, D. (2008). Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes and Development, 22(16), 2189–2203. https://doi.org/10.1101/gad.1700908
Lantoine, J., Grevesse, T., Villers, A., Delhaye, G., Mestdagh, C., Versaevel, M., … Gabriele, S. (2016). Matrix stiffness modulates formation and activity of neuronal networks of controlled architectures. Biomaterials, 89, 14–24. https://doi.org/10.1016/j.biomaterials.2016.02.041
Levina, E. M., Kharitonova, M. a, Rovensky, Y. a, & Vasiliev, J. M. (2001). Cytoskeletal control of fibroblast length: experiments with linear strips of substrate. Journal of Cell Science, 114, 4335–4341.
Li, R., & Bruce, B. (2009). Symmetry Breaking in Biology, 4(3), 1521–1528. https://doi.org/10.1101/cshperspect.a003475
Li, Y., Schnekenburger, J., & Duits, M. H. G. (2012). Intracellular particle tracking as a tool for tumor cell characterization. Journal of Biomedical Optics, 14(6), 64005. https://doi.org/10.1117/1.3257253
Mahmud, G., Campbell, C. J., Bishop, K. J. M., Komarova, Y. a., Chaga, O., Soh, S., … Grzybowski, B. a. (2009). Directing cell motions on micropatterned ratchets. Nature Physics, 5(8), 606–612. https://doi.org/10.1038/nphys1306
Mandal, K., Asnacios, A., Goud, B., & Manneville, J.-B. (2016). Mapping intracellular mechanics on micropatterned substrates. Proceedings of the National Academy of Sciences of the United States of America, 113(46), E7159–E7168. https://doi.org/10.1073/pnas.1605112113
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K., & Chen, C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell, 6(4), 483–495. https://doi.org/10.1016/S1534-5807(04)00075-9
Park, T. H., & Shuler, M. L. (2003). Integration of cell culture and microfabrication technology. Biotechnology Progress, 19(2), 243–253. https://doi.org/10.1021/bp020143k
Pitaval, A., Tseng, Q., Bornens, M., & Théry, M. (2010). Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. Journal of Cell Biology, 191(2), 303–312. https://doi.org/10.1083/jcb.201004003
Polte, T. R. (2004). Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. AJP: Cell Physiology, 286(3), 518C–528. https://doi.org/10.1152/ajpcell.00280.2003
Pouthas, F., Girard, P., Lecaudey, V., Ly, T. B. N., Gilmour, D., Boulin, C., … Reynaud, E. G. (2008). In migrating cells, the Golgi complex and the position of the centrosome depend on geometrical constraints of the substratum. Journal of Cell Science, 121(Pt 14), 2406–2414. https://doi.org/10.1242/jcs.026849
Roca-Cusachs, P., Alcaraz, J., Sunyer, R., Samitier, J., Farré, R., & Navajas, D. (2008). Micropatterning of Single Endothelial Cell Shape Reveals a Tight Coupling between Nuclear Volume in G1 and Proliferation. Biophysical Journal, 94(12), 4984–4995. https://doi.org/10.1529/biophysj.107.116863
Rolli, C. G., Nakayama, H., Yamaguchi, K., Spatz, J. P., Kemkemer, R., & Nakanishi, J. (2012). Switchable adhesive substrates: Revealing geometry dependence in collective cell behavior. Biomaterials, 33(8), 2409–2418. https://doi.org/10.1016/j.biomaterials.2011.12.012
Roth, S., Bisbal, M., Brocard, J., Bugnicourt, G., Saoudi, Y., Andrieux, A., … Villard, C. (2012). How morphological constraints affect axonal polarity in mouse neurons. PLoS ONE, 7(3), 1–9. https://doi.org/10.1371/journal.pone.0033623
Schwenk, J. M., Stoll, D., Templin, M. F., & Joos, T. O. (1983). Biotechniques. BioTechniques. [Eaton Pub. Co.]. Retrieved from http://cat.inist.fr/?aModele=afficheN&cpsidt=15272695
Segerer, F. J., Th??roff, F., Piera Alberola, A., Frey, E., & R??dler, J. O. (2015). Emergence and persistence of collective cell migration on small circular micropatterns. Physical Review Letters, 114(22), 1–5. https://doi.org/10.1103/PhysRevLett.114.228102
Singhvi, R., Kumar, A., Lopez, G. P., Stephanopoulos, G. N., Wang, D. I. C., Whitesides, G. M., & Ingber, D. E. (1994). Engineering cell shape and function. Science, 264(5159), 696–698. Retrieved from http://www.scopus.com/inward/record.url?eid=2-s2.0-0028338446&partnerID=tZOtx3y1
Tan, J. L., Tien, J., Pirone, D. M., Gray, D. S., Bhadriraju, K., & Chen, C. S. (2003). Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America, 100(4), 1484–9. https://doi.org/10.1073/pnas.0235407100
Théry, M., & Bornens, M. (2006). Cell shape and cell division. Current Opinion in Cell Biology, 18(6), 648–657. https://doi.org/10.1016/j.ceb.2006.10.001
Théry, M., Racine, V., Pépin, A., Piel, M., Chen, Y., Sibarita, J.-B., & Bornens, M. (2005). The extracellular matrix guides the orientation of the cell division axis. Nature Cell Biology, 7(10), 947–953. https://doi.org/10.1038/ncb1307
Tseng, P., Kunze, A., Kittur, H., & Di Carlo, D. (2014). Research highlights: microtechnologies for engineering the cellular environment. Lab on a Chip, 14(7), 1226. https://doi.org/10.1039/c4lc90012j
Versaevel, M., Grevesse, T., & Gabriele, S. (2012). Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nature Communications, 3, 671. https://doi.org/10.1038/ncomms1668
Vogt, A. K., Wrobel, G., Meyer, W., Knoll, W., & Offenhäusser, A. (2005). Synaptic plasticity in micropatterned neuronal networks. Biomaterials, 26(15), 2549–2557. https://doi.org/10.1016/j.biomaterials.2004.07.031
Wan, L. Q., Ronaldson, K., Park, M., Taylor, G., Zhang, Y., Gimble, J. M., & Vunjak-Novakovic, G. (2011). Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proceedings of the National Academy of Sciences of the United States of America, 108(30), 12295–12300. https://doi.org/10.1073/pnas.1103834108
Wyart, C., Ybert, C., Bourdieu, L., Herr, C., Prinz, C., & Chatenay, D. (2002). Constrained synaptic connectivity in functional mammalian neuronal networks grown on patterned surfaces. Journal of Neuroscience Methods, 117(2), 123–131. https://doi.org/10.1016/S0165-0270(02)00077-8
Yamamoto, H., Demura, T., Morita, M., Banker, G. A., Tanii, T., & Nakamura, S. (2012). Differential neurite outgrowth is required for axon specification by cultured hippocampal neurons. Journal of Neurochemistry, 123(6), 904–910. https://doi.org/10.1111/jnc.12001
Yamamoto, H., Matsumura, R., Takaoki, H., Katsurabayashi, S., Hirano-Iwata, A., & Niwano, M. (2016). Unidirectional signal propagation in primary neurons micropatterned at a single-cell resolution. Applied Physics Letters, 109(4). https://doi.org/10.1063/1.4959836
Yang, I. H., Co, C. C., & Ho, C.-C. (2005). Alteration of human neuroblastoma cell morphology and neurite extension with micropatterns. Biomaterials, 26(33), 6599–6609. https://doi.org/10.1016/j.biomaterials.2005.04.024
Yoshida, S., Teshima, T., Kuribayashi-Shigetomi, K., & Takeuchi, S. (2016). Mobile Microplates for Morphological Control and Assembly of Individual Neural Cells. Advanced Healthcare Materials, 5(4), 415–420. https://doi.org/10.1002/adhm.201500782
Zhang, H., Hou, R., Xiao, P., Xing, R., Chen, T., Han, Y., … Fu, J. (2016). Single cell migration dynamics mediated by geometric confinement. Colloids and Surfaces B: Biointerfaces, 145, 72–78. https://doi.org/10.1016/j.colsurfb.2016.04.039
Ziegler, C. (1999). Cell-based biosensors. Trends in Biotechnology, 3(4), 92–96. https://doi.org/10.1016/0167-7799(85)90091-5